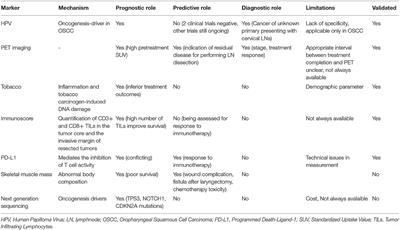
Frontiers | Diagnostic Tumor Markers in Head and Neck Squamous Cell Carcinoma (HNSCC) in the Clinical Setting
Outcomes of patients presenting with elevated tumor marker levels but negative gadoxetic acid-enhanced liver MRI after a complete response to hepatocellular carcinoma treatment | PLOS ONE

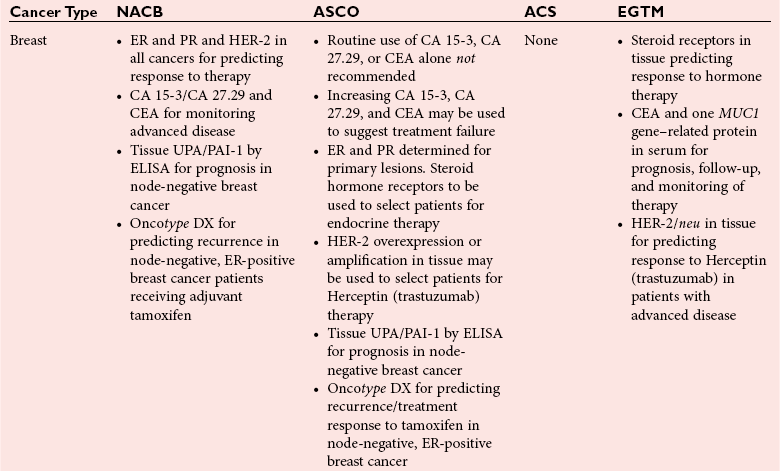
Clinical use of biomarkers in breast cancer: Updated guidelines from the European Group on Tumor Markers (EGTM) – topic of research paper in Clinical medicine. Download scholarly article PDF and read for

2007 UPDATE OF ASCO RECOMMENDATIONS FOR THE USE OF TUMOR MARKERS IN BREAST CANCER Clinical Practice Guideline. - ppt download
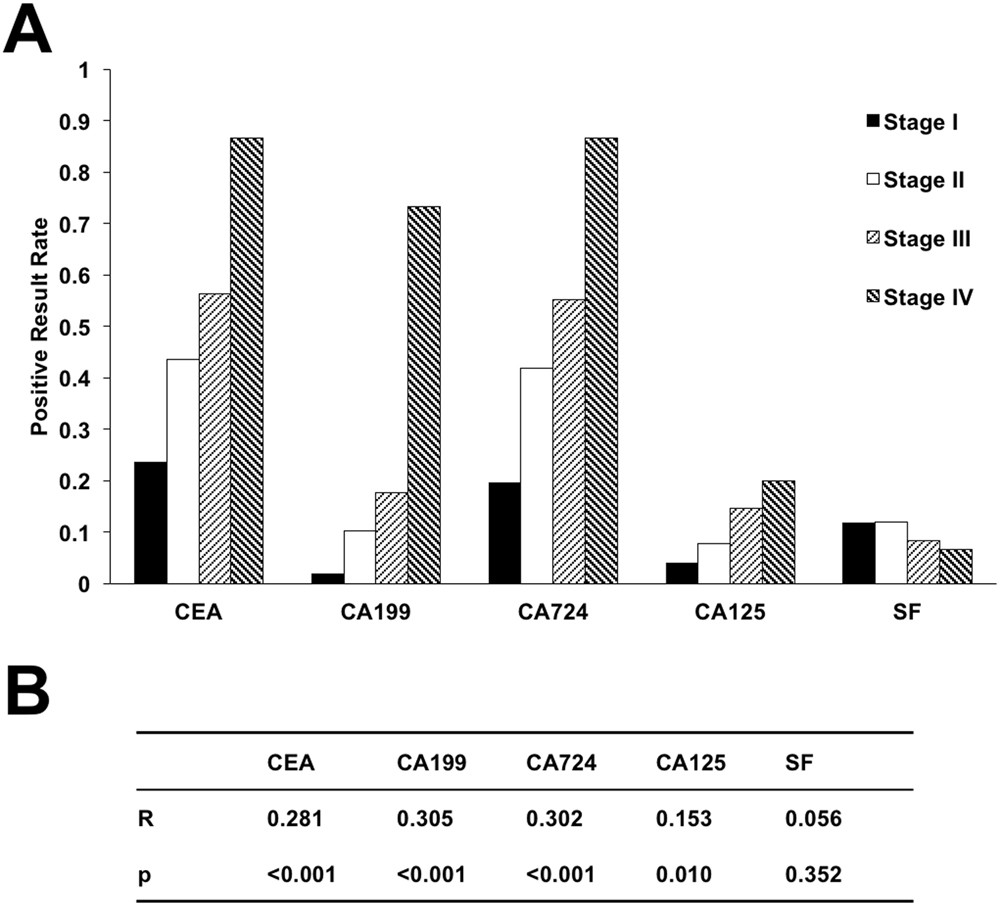
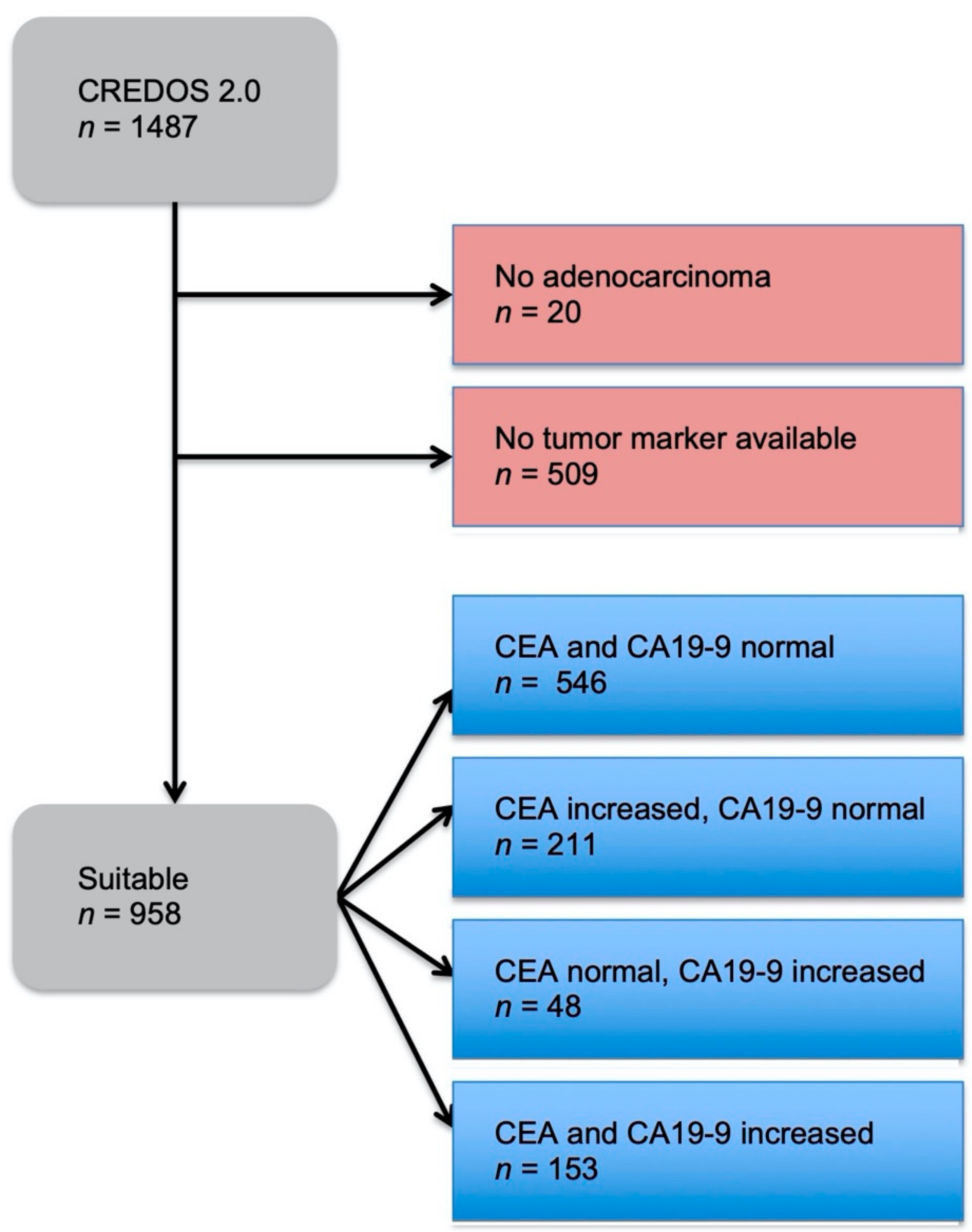
Evaluation of Serum CEA, CA19-9, CA72-4, CA125 and Ferritin as Diagnostic Markers and Factors of Clinical Parameters for Colorectal Cancer | Scientific Reports
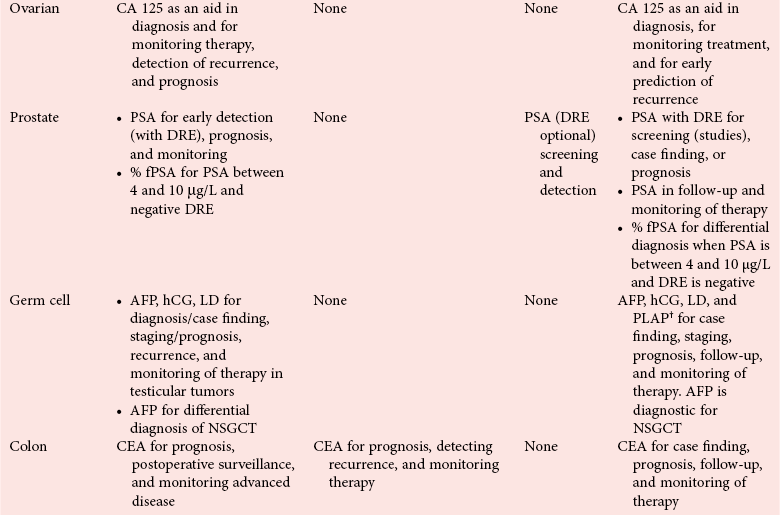
![PDF] National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for the Use of Tumor Markers. | Semantic Scholar PDF] National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for the Use of Tumor Markers. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/852dcccd618ad917d7cc0ce18504c982c1708b79/4-Table3-1.png)
PDF] National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for the Use of Tumor Markers. | Semantic Scholar
![PDF] National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for the Use of Tumor Markers. | Semantic Scholar PDF] National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for the Use of Tumor Markers. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/852dcccd618ad917d7cc0ce18504c982c1708b79/3-Table2-1.png)
PDF] National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for the Use of Tumor Markers. | Semantic Scholar
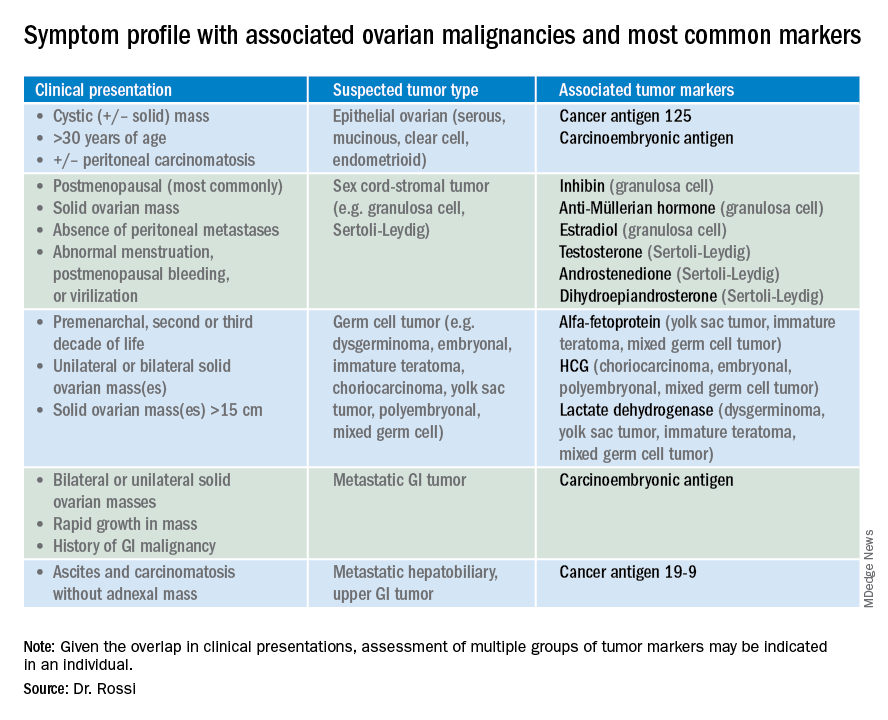
![PDF] National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. | Semantic Scholar PDF] National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e838d88fd75ebe4c94deae98769ba9a385403e5e/4-Table1-1.png)
PDF] National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. | Semantic Scholar